Research Article
Utilization Rate of Helicobacter pylori
Immunohistochemistry Is Not Associated With
the Diagnostic Rate of Helicobacter pylori Infection
Jung H. Son, MD,* Benjamin Lebwohl, MD, MS,† Antonia R. Sepulveda, MD, PhD,* and Stephen M. Lagana, MD*
Background: Utilization rates of immunohistochemistry (IHC) for the diagnosis of Helicobacter pylori infection may vary by laboratory and/or pathologists. IHC for H. pylori is not performed routinely in our practice. Instead, it is used in selected cases at the pathologists’ discretion (and according to their specific criteria). The purpose of this study was to determine if IHC utilization rates correlated with rates of detecting H. pylori infection.
Materials and Methods: We searched our records and investigated all gastric biopsies for 1 calendar year. H. pylori diagnostic rate and IHC utilization rate was calculated for each pathologist.
Results: Overall, the rate of diagnosis was 12.1% and the IHC utilization rate was 45.2%. Individual pathologists had H. pylori diagnostic rates ranging from 3.6% to 34.1% (median: 11.1%) and IHC utilization ranging from 17.1% to 95.2% (median:
42.2%). The rate of detection of H. pylori infection among pathologists showed no significant correlation with rates of IHC utilization (Pearson coefficient= 0.121).
Conclusions: Increasing use of IHC is not independently associated with the diagnostic rate of infection. Ultimately, if we assume that the case mix was similar for each pathologist, it suggests that more liberal criteria to order IHC does not result in
more infections diagnosed.
Key Words: Helicobacter pylori, H. pylori, gastritis, immunohistochemistry, IHC, gastrointestinal pathology, diagnostic rate, IHC utilization
(Appl Immunohistochem Mol Morphol 2019;27:694–698)
____________________________________________________
Received for publication February 13, 2018; accepted April 18, 2018.
From the *Department of Pathology and Cell Biology; and †Celiac Disease Center, Columbia University Medical Center, New York, NY.
J.H.S. collected and compiled the data and drafted the manuscript. B.L. participated in the design of the study and performed the statistical analysis. A.R.S. participated in the design of the study and provided
critical revisions. S.M.L. conceived the study, and participated in its design and coordination and helped to draft the manuscript.
The authors declare no conflict of interest.
Reprints: Stephen M. Lagana, MD, Columbia University Medical Center, 622 W 168th St, Rm VC15-202, New York, NY 10032
(e-mail: sml2179@cumc.columbia.edu).
Copyright © 2018 Wolters Kluwer Health, Inc. All rights reserved
Helicobacter pylori is one of the most common bacterial infections worldwide, estimated to have infected ~50% of the world1 and identified in 12% of gastric biopsies in the United States.2 First identified as a pathogenic organism for peptic ulcers by Marshall and Warren3 in 1982, H. pylori is a gram-negative rod that is either spiral or comma-shaped
and measures 2.4 to 4.0 µm.1,4 Since its discovery and identification, numerous studies have been published, firmly establishing the association of H. pylori with chronic gastritis, peptic ulcers, and gastric malignancy.3,5,6 Because of these associations, there is great pressure on both clinicians and pathologists to accurately identify and treat patients
with H. pylori infection.7
Current testing approaches for H. pylori include routine histology with or without special stains, rapid urease testing, molecular testing with polymerase chain reaction (PCR), serological antibody testing, urea breath tests, and fecal antigen test.8,9 Although there is no defined consensus with regard to the “gold standard” testing protocol for the diagnosis of H. pylori infection, endoscopic gastric biopsies with histologic evaluation is generally accepted as a good
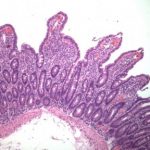
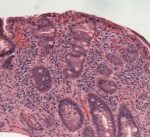
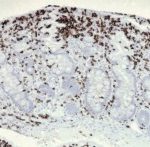
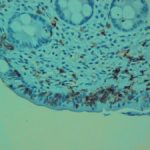
method of diagnosing H. pylori and related pathologies. The major advantage of endoscopic biopsy-based histologic evaluation is that it provides near perfect sensitivity and specificity for the detection of H. pylori organisms.7,8 An additional benefit is the possibility of diagnosing other inflammatory and neoplastic conditions (either distinct from, or as a consequence of H. pylori infection). Careful microscopic evaluation of routine hematoxylin and eosin (H&E)-stained preparation is usually sufficient to identify H. pylori organisms.10,11 However, a variety of ancillary special stains (eg, Giemsa, Warthin-Starry, acridine orange, toluidine blue, Alcian blue, Diff-Quik) and immunohistochemical (IHC) antibodies against H. pylori are available to further aid the
identification of H. pylori bacteria in tissue sections.8–12
These ancillary tests provide benefit in some situations, but their availability raises questions. First, should all gastric biopsy cases be subjected to ancillary testing, and if so which test, and if not, which cases should be tested?
The convenience, quicker turn-around time, and putative ability to capture more positive cases, along with (potential) financial incentives in a fee-for-service environment have led many practices to adopt protocol “up-front” ancillary testing. The major downside to up-front staining is that it may be unnecessarily increasing the overall
costs associated with a pathologist’s interpretation of a gastric biopsy.
Batts et al8 provided guidelines for the use of H. pylori ancillary testing based on expert consensus among members of the Rodger C. Haggitt Gastrointestinal Pathology Society. The major point the authors emphasize is the fact that the vast majority of H. pylori infections can be diagnosed on
H&E stain with high sensitivity and specificity of 91% to 100%.9,13,14 The group recommended judicious use of IHC based on histologic findings and strongly recommends against up-front testing for H. pylori organisms. Chronic active gastritis (lymphoplasmacytic and neutrophilic inflammation) in which H. pylori organisms are not detected by routine stain is a definite indication for ancillary testing.
The authors further suggest testing in chronic inactive gastritis with at least moderate gastritis.8 Unfortunately, the distinction between mild chronic gastritis, without the need for further testing, and moderate chronic gastritis which should be tested, is incompletely defined. In contrast, the Houston update to the Sydney classification of gastritis seemed to suggest testing all gastric biopsies, to ensure a
careful examination and due to perceived efficiency benefits.
At other points in the manuscript, the authors made the recommendation somewhat more targeted by saying staining should be performed in “inflamed” biopsies in which H. pylori could not be detected by H&E.15 This group discussed the limitations in diagnosing inactive chronic gastritis, including an imperfect definition of normalcy, geographic variation in gastric immune cell concentrations, and variably applied pathologic criteria.15 Given these tremendous obstacles to a precisely defined abnormal state, it is not surprising that a practical point of distinction between “mild chronic inactive gastritis” and “moderate chronic inactive gastritis” was not offered.
At our institution, the use of ancillary testing for H. pylori is at the discretion of the attending pathologist. As a practical matter, special histochemical stains are rarely, if ever, used by our pathologists for this purpose, whereas the immunohistochemical stain (anti-H. pylori antibody; BioCare Medical, LLC, Concord, CA) is the default ancillary stain when one is considered necessary. No reflexive testing is performed and no strictly enforced rules are in place with regard to when an IHC stain should be ordered. Individual pathologists have no financial incentives to use or not use IHC. Thus, we hypothesized that there would be variability in H. pylori IHC usage rates among pathologists. We further hypothesized that since most pathologists would see a similar case mix, the difference in utilization rates would most likely be the result of varying criteria for testing. We assumed that different pathologists would have different thresholds for when an immunohistochemical test was required. We believe
this to be a reasonable assumption due to the lack of broadly accepted diagnostic criteria and testing algorithms, as discussed above. On the basis of this assumption, we determined if those pathologists who used IHC more frequently had a higher H. pylori diagnostic rate than those using IHC less frequently. We also investigated if pathologists with subspecialty practice in gastrointestinal (GI) pathology used IHC
at a different rate than general surgical pathologists
MATERIALS AND METHODS
Following approval by the Columbia University
Institutional Review Board [IRB-AAAO2353 (Y1M00)],
all gastric biopsy pathology reports from the New York Presbyterian/Columbia University Medical Center during the calendar year 2013 were retrieved and retrospectively reviewed. The initial database query selected for any cases that had a part-type text “stomach,” a term that is always included in specimen part-type assignment of stomach biopsies. Cases accessioned with multiple parts were included, as long at least 1 part of the case was from the
stomach. Gastroesophageal junction and gastric cardia specimens were excluded as biopsies at these sites are commonly performed for evaluation of reflux-related pathologies and H. pylori is less frequently diagnosed in these sites.
Resulting data consisted of the case number, case
pathologist, part-type information, final diagnosis, and
comments/notes section texts queried from the Cerner CoPath Plus database. This was then tabulated onto a spreadsheet for data review. For each case, we reviewed the final diagnosis text, comments, and addenda (if applicable) in order to tally the following: (1) the number of gastric biopsies in the case, (2) the number of H. pylori positive biopsies, (3) the number of IHC stains ordered,
and (4) the number of positive IHC stains.
These annotated data were imported as a comma separated values file into RStudio with R version 3.2.0 (64-bit) to facilitate data manipulation and statistical analyses. We calculated the diagnostic rate of H. pylori positive cases and the number of IHC stains ordered on a per case basis. For our subgroup analysis, the IHC usage rate and diagnostic rates of H. pylori of GI versus general surgical pathologists were compared. At our institution, GI biopsies are divided between a subspecialized GI pathology service and a general surgical pathology service according to a loadbalancing system that depends dynamically on several factors including daily case volume and optimization of resident education. We defined a GI pathologist (n=8) as a faculty member who has completed a GI pathology fellowship and who rotates on the subspecialized service.
Statistical Methods
Using R software, we calculated the Pearson product moment correlation coefficient to determine if diagnostic rate and IHC utilization rates among pathologists significantly correlated. The Welch t test was used to measure differences between the mean diagnostic rates and the mean IHC utilization rates within the GI versus general surgical pathologists subgroups.
RESULTS
Distribution of Cases and Pathologists
A total of 3751 cases matching our selection criteria were identified dispersed among 19 pathologists (Table 1). The median total case load was 127 and ranged from 41 to 518 per attending pathologist. GI pathologists (n=8) had a median of 311 cases and a range of 42 to 518 cases and 11 general surgical pathologists had a median of 120 cases and a range of
ARTICLE HISTORY
Received 20 June 2018
Revised 31 July 2018
Accepted 4 August 2018
KEYWORDS
Celiac disease; endocrine manifestations; hypothyroidism; osteoporosis
ABSTRACT
Purpose: The purpose of this article is to review recent literature regarding endocrine disorders related to celiac disease (CD).
Methods: We describe a case report and review existing literature on the endocrine manifestations of CD.
Results: CD is an autoimmune disorder characterized by intestinal inflammation in response to gluten. CD can cause a wide range of extra-intestinal complications, including endocrine manifestations. Metabolic bone disease including osteoporosis and osteopenia, vitamin D deficiency, secondary hyperparathyroidism and less frequently osteomalacia can be seen. In CD, fracture risk is increased by 30–40%, while risk for hip fracture is approximately doubled. The risk for other endocrine disorders, particularly autoimmune endocrinopathies, is also increased in those with CD compared to the general population. Epidemiologic data indicate the risk for hypothyroidism is 3–4 times higher among those with CD, while risk of type 1 diabetes is greater than double. Risk for primary adrenal insufficiency is a striking 11-fold higher in those with versus without CD, though the absolute risk is low. Fertility is reduced in women with CD before diagnosis by 37% while male fertility in the absence of hypogonadism does not appear to be affected. Other endocrine conditions including hyperthyroidism, ovarian failure, androgen insensitivity, impaired growth and growth hormone deficiency and autoimmune polyendocrine syndromes have also been associated with CD.
Conclusions: CD is associated with a wide range of endocrine manifestations.
CD is an autoimmune disorder characterized by intestinal inflammation in response to gluten, a family of proteins found in wheat, barley, and rye. 1 Classic CD is characterized by malabsorption, diarrhea, and weight loss, though today many patients have only minor gastrointestinal symptoms and can be asymptomatic. CD can also cause a wide range of extra-intestinal complications, including endocrine manifestations. 2 Metabolic bone disease including osteoporosis and osteopenia, vitamin D deficiency, secondary hyperparathyroidism and less frequently osteomalacia can be seen. CD is also associated with other endocrine disorders including, hypo- and hyperthyroidism, type 1 diabetes, adrenal insufficiency, ovarian failure and infertility, androgen insensitivity, impaired growth and growth hormone deficiency (GHD) and autoimmune polyendocrine syndromes. This review will summarize endocrine disorders related to CD with a focus on recent investigations where available.
Methods
We describe a case report of a patient with CD and multiple endocrine comorbidities and review the existing literature of the endocrine manifestations of CD.
Case report
A 60-year-old man presented for endocrine evaluation due to a 4-year history of fatigue, reduced muscle mass and strength, loss of libido, mild cognitive changes, depressive symptoms, and weight loss of 20 pounds. Prior initial outside evaluation indicated an elevated total testosterone level. The patient reported loss of appetite and constipation but no vomiting, diarrhea, abdominal pain, or food intolerance. Colonoscopy, 3 years prior, had been normal. He was the biological father of two sons ages 30 and 27 and reported no other symptoms of hypogonadism. He described one inch of height loss without history of fracture and did not smoke or consume alcohol. He had been on varying antiepileptic drugs for 30 years following a generalized tonic-clonic seizure associated with a right temporal subarachnoid cyst, which was stable on serial imaging. At presentation, his medications included divalproex and vitamin D. The patient denied theuse of other medications. On examination, the patient was 67.5 kilograms and euthyroid-appearing with a normal adult male phenotype and body mass index (BMI) 22.1 kg/m 2. He did not meet criteria for anorexia. The thyroid gland was normal in size with irregular texture. There was no kyphosis or gynecomastia. Abdominal exam was normal. Circumcised phallus and testes w
| Normal Range | Initial Evaluation Pretreatment | 1-year after Testosterone Treatment | After GFD Initiation on Testosterone | On GFD and off Testosterone | |
| Serum Biochemistries, Renal function, Liver function | |||||
| Sodium (mmol/L) | 135–145 | 132, 138 | |||
| Potassium (mmol/L) | 3.3–5.0 | 4.2 | |||
| Metabolic Bone Evaluation | |||||
| Sodium (mmol/L) | 135–145 | 132, 138 | 9.5 | ||
| Potassium (mmol/L) | 3.3–5.0 | 4.2 | |||
Digestive Diseases and Sciences
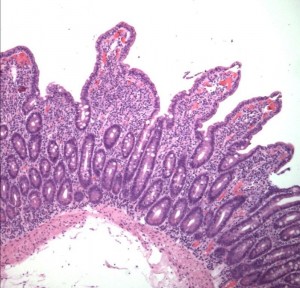
Celiac Disease Pathogenesis III 300×288
Treatment and ResponseTreatment data were available in 32 of 33 (97%) cases, and clinical response was documented in 30. Of 30, 24 (80%) showed a clear clinical response to antibiotic therapy, 2 of 30 (7%) no clinical response or further deterioration, and 4 of 30 (13%) equivocal clinical response after median follow-up of 14 months (IQR 2–39). Two of the four cases with equivocal clinical response were CNS disease treated with intravenous (IV) ceftriaxone and either oral trimetho-prim–sulfamethoxazole (TMP-SMX) or ceftibuten, and the two other cases were classic WD treated with ceftriaxone and TMP-SMX. The majority, 23 of 32 (72%), received a combination of IV ceftriaxone and oral TMP-SMX. TMP-SMX monotherapy was used in 7 of 32 (25%), minocycline monotherapy in 1 of 32 (4%), and 1 patient deferred ther-apy. Of the MCR cohort, 4 of 28 cases had serum levels of antibiotics followed.Lack of response to therapy or further deterioration occurred in two patients, one with classic WD and the other in the patient that deferred therapy. The lack of response in the classic WD case may represent immune reconstitution inflammatory syndrome (IRIS) as opposed to treatment failure, due to the initial response to pred-nisone; however, there was no confirmation of successful treatment through a negative tissue PCR, which is required for diagnosis of IRIS [31].All cases treated with T
Digestive Diseases and Sciences

Celiac Disease Pathogenesis III 300×288
Treatment and ResponseTreatment data were available in 32 of 33 (97%) cases, and clinical response was documented in 30. Of 30, 24 (80%) showed a clear clinical response to antibiotic therapy, 2 of 30 (7%) no clinical response or further deterioration, and 4 of 30 (13%) equivocal clinical response after median follow-up of 14 months (IQR 2–39). Two of the four cases with equivocal clinical response were CNS disease treated with intravenous (IV) ceftriaxone and either oral trimetho-prim–sulfamethoxazole (TMP-SMX) or ceftibuten, and the two other cases were classic WD treated with ceftriaxone and TMP-SMX. The majority, 23 of 32 (72%), received a combination of IV ceftriaxone and oral TMP-SMX. TMP-SMX monotherapy was used in 7 of 32 (25%), minocycline monotherapy in 1 of 32 (4%), and 1 patient deferred ther-apy. Of the MCR cohort, 4 of 28 cases had serum levels of antibiotics followed.Lack of response to therapy or further deterioration occurred in two patients, one with classic WD and the other in the patient that deferred therapy. The lack of response in the classic WD case may represent immune reconstitution inflammatory syndrome (IRIS) as opposed to treatment failure, due to the initial response to pred-nisone; however, there was no confirmation of successful treatment through a negative tissue PCR, which is required for diagnosis of IRIS [31].All cases treated with T
Digestive Diseases and Sciences

Celiac Disease Pathogenesis III 300×288
Treatment and ResponseTreatment data were available in 32 of 33 (97%) cases, and clinical response was documented in 30. Of 30, 24 (80%) showed a clear clinical response to antibiotic therapy, 2 of 30 (7%) no clinical response or further deterioration, and 4 of 30 (13%) equivocal clinical response after median follow-up of 14 months (IQR 2–39). Two of the four cases with equivocal clinical response were CNS disease treated with intravenous (IV) ceftriaxone and either oral trimetho-prim–sulfamethoxazole (TMP-SMX) or ceftibuten, and the two other cases were classic WD treated with ceftriaxone and TMP-SMX. The majority, 23 of 32 (72%), received a combination of IV ceftriaxone and oral TMP-SMX. TMP-SMX monotherapy was used in 7 of 32 (25%), minocycline monotherapy in 1 of 32 (4%), and 1 patient deferred ther-apy. Of the MCR cohort, 4 of 28 cases had serum levels of antibiotics followed.Lack of response to therapy or further deterioration occurred in two patients, one with classic WD and the other in the patient that deferred therapy. The lack of response in the classic WD case may represent immune reconstitution inflammatory syndrome (IRIS) as opposed to treatment failure, due to the initial response to pred-nisone; however, there was no confirmation of successful treatment through a negative tissue PCR, which is required for diagnosis of IRIS [31].
Treatment and ResponseTreatment data were available in 32 of 33 (97%) cases, and clinical response was documented in 30. Of 30, 24 (80%) showed a clear clinical response to antibiotic therapy, 2 of 30 (7%) no clinical response or further deterioration, and 4 of 30 (13%) equivocal clinical response after median follow-up of 14 months (IQR 2–39). Two of the four cases with equivocal clinical response were CNS disease treated with intravenous (IV) ceftriaxone and either oral trimetho-prim–sulfamethoxazole (TMP-SMX) or ceftibuten, and the two other cases were classic WD treated with ceftriaxone and TMP-SMX. The majority, 23 of 32 (72%), received a combination of IV ceftriaxone and oral TMP-SMX. TMP-SMX monotherapy was used in 7 of 32 (25%), minocycline monotherapy in 1 of 32 (4%), and 1 patient deferred ther-apy. Of the MCR cohort, 4 of 28 cases had serum levels of antibiotics followed.Lack of response to therapy or further deterioration occurred in two patients, one with classic WD and the other in the patient that deferred therapy. The lack of response in the classic WD case may represent immune reconstitution inflammatory syndrome (IRIS) as opposed to treatment failure, due to the initial response to pred-nisone; however, there was no confirmation of successful treatment through a negative tissue PCR, which is required for diagnosis of IRIS [31].All cases treated with T
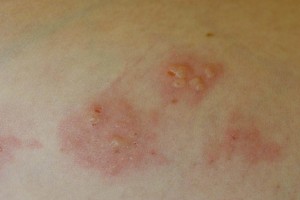
Celiac Disease Definition and Clinical Manifestations Dermatitis Herpetiformis 300×200
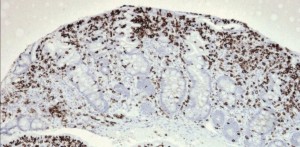
Celiac Disease Epidemiology poorly responsive II 300×147
Digestive Diseases and Sciences
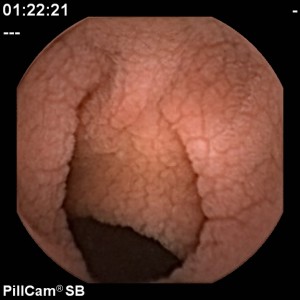
Celiac Disease Diagnosis video capsule 300×300
Treatment and ResponseTreatment data were available in 32 of 33 (97%) cases, and clinical response was documented in 30. Of 30, 24 (80%) showed a clear clinical response to antibiotic therapy, 2 of 30 (7%) no clinical response or further deterioration, and 4 of 30 (13%) equivocal clinical response after median follow-up of 14 months (IQR 2–39). Two of the four cases with equivocal clinical response were CNS disease treated with intravenous (IV) ceftriaxone and either oral trimetho-prim–sulfamethoxazole (TMP-SMX) or ceftibuten, and the two other cases were classic WD treated with ceftriaxone and TMP-SMX. The majority, 23 of 32 (72%), received a combination of IV ceftriaxone and oral TMP-SMX. TMP-SMX monotherapy was used in 7 of 32 (25%), minocycline monotherapy in 1 of 32 (4%), and 1 patient deferred ther-apy. Of the MCR cohort, 4 of 28 cases had serum levels of antibiotics followed.Lack of response to therapy or further deterioration occurred in two patients, one with classic WD and the other in the patient that deferred therapy. The lack of response in the classic WD case may represent immune reconstitution inflammatory syndrome (IRIS) as opposed to treatment failure, due to the initial response to pred-nisone; however, there was no confirmation of successful treatment through a negative tissue PCR, which is required for diagnosis of IRIS [31].All cases treated with T